Synthesis of Hydrobromic Acid
Preparation of Hydrobromic Acid
Hydrobromic Acid: Of the six methods given here, the first three may be used for the production of gaseous hydrogen bromide as well as for aqueous solutions of the gas; rest of them could be employed only for making the constant-boiling hydrobromic acid acid.
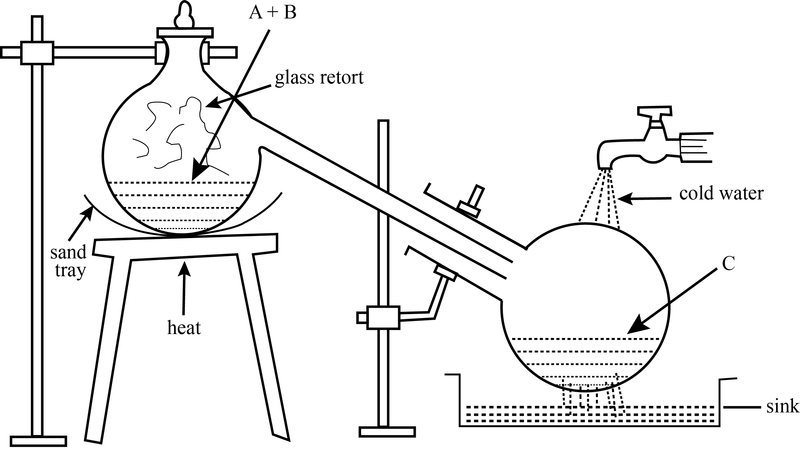
The apparatus for hydrobromic acid is made of a gas-washing bottle filled with water, connected to a distilling flask filled with 50 ml of bromine cooled in ice with the inlet tube leading almost to the bottom of the flask. The inlet tube is connected to a Pyrex tube, 50 cm long, packed with about 30 cm of activated charcoal which is held in place with glass wool plugs.
The outlet tube connected to a U-tube filled with glass beads or porcelain chips coated with moist red phosphorus. Finally two wash bottles in series filled with 100 ml and 50 ml of water, respectively; if constant-boiling acid is desired, the second bottle should contain 100 ml of water. Both bottles are surrounded by an ice-salt bath.
A steady flow of hydrogen is started through the apparatus to displace the air. This operation requires about thirty minutes, during which time the bromine is kept in the cooling bath. The furnace is now slowly warmed to 350-375° C and the flow of hydrogen is increased to a point where the bubbles are just too rapid to count. A bath of water at 40-45°C is now placed under the vessel containing the bromine; under these conditions, conversion to hydrogen bromide is complete in about 20 minutes.
No vapors of bromine should be visible at the outlet of the reaction tube; otherwise the bromine must be cooled somewhat in water. At the end of the experiment the two wash bottles are removed and the hydrobromic acid absorbed is determined from the gain in weight; if the smaller volume of water was used, the concentration of acid should be 60-65% by weight.
If constant-boiling acid is desired, the solution should be distilled and the fraction boiling at 122-127 °C collected as 47-48% hydrobromic acid. A rapid method for determining the concentration of the solution produced is by a rough determination of specific gravity. Yield 90%.
In a distilling flask fitted with a dropping funnel are placed 25 g of clean sand and, over this, a mixture of 25 g of red phosphorus and 100 g of sand. The mass is moistened with 40-45 ml of water, and 50 ml of bromine are introduced into the funnel. The outlet tube connected to a U-tube filled with glass beads or porcelain chips coated with moist red phosphorus. Finally two wash bottles in series filled with 100 ml and 50 ml of water, respectively; if constant-boiling acid is desired, the second bottle should contain 100 ml of water.
Both bottles are surrounded by an ice-salt bath. The reaction flask is cooled in ice while the bromine is added dropwise at a very slow rate; the phosphorus may glow with each addition of halogen. In order to avoid a suck-back of the water in the absorption flasks, it is advisable to place an empty safety trap between the U-tube and the water traps.
As the reaction proceeds, the evolution of gas is more readily controlled and the cooling bath may be removed. When all the bromine has been added, the distilling flask is gently warmed to drive off the remaining acid vapors. The working-up of the hydrobromic acid solutions is the same as previously described. Yield 90%.
Thirty-five grams of tetrahydronaphthalene are placed in the flask with either 200 ml of water, depending on the desired concentration of the acid. At first the flask is cooled in ice while 50 ml of bromine are slowly added drop-wise ; about one gram of iron filings catalyzes the bromination.
As the reaction proceeds, the flask may be allowed to warm up to room temperature. After all the bromine has been added the flask should be shaken for some time; the aqueous layer should be colorless. The acid layer is then separated from the organic material and worked up as in “Preparation of hydrobromic acid directly from hydrogen and bromine“.
A mixture of 120 g of potassium bromide and 200 ml of water is cooled in ice while 90 ml of concentrated sulfuric acid is slowly added. The temperature must not rise over 75° C during this addition; otherwise free bromine may be formed, causing a loss in yield.
The reaction mixture is cooled to room temperature and the potassium bisulfate is filtered off by suction through a fritted funnel or a hardened filter paper. The filtrate is then fractionated and the material boiling from 122-127° C is collected as constant-boiling acid. Yield 85%.
In all cases where a mixture of sulfuric and hydrobromic acids is obtained, a redistillation is necessary to remove about 0.01% of sulfate in the first fractionation; only the acid with a steady boiling point is retained. This operation entails a loss of about 15% in yield.
Fifty milliliters of bromine are covered with 200 ml of water and sulfur dioxide is passed into the mixture, under the hood, until a straw-colored liquid results. Fractionation yields about 300 g of 47-48% acid, which is an almost theoretical yield. As this reaction proceeds, the bromine dissolves in the hydrobromic acid that is formed, yielding a homogeneous liquid into which the sulfur dioxide may be more rapidly introduced.