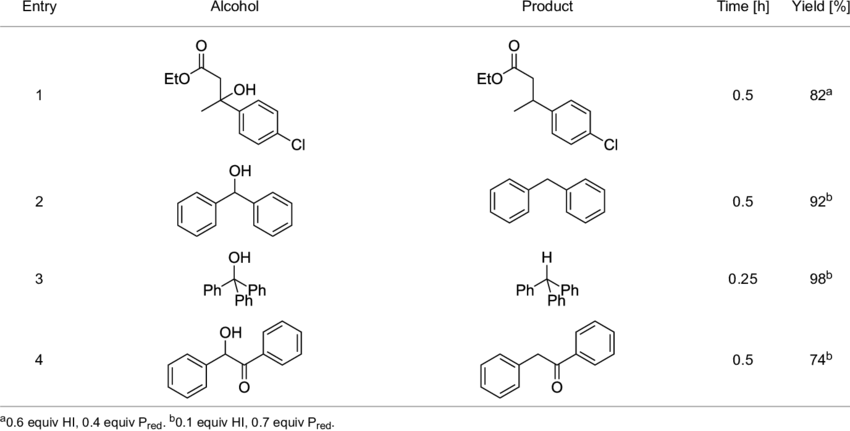
Synthesis of Hydroiodic Acid
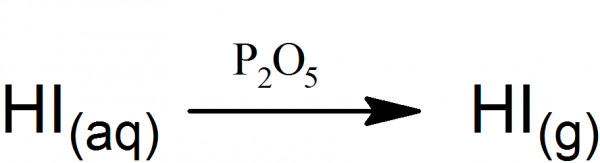
Preparation of Hydroiodic Acid
Hydroiodic Acid: Rubber stoppers, as well as direct light, should be avoided in all operations with iodine, hydrogen iodide and hydroiodic acid. Platinized asbestos is prepared by soaking 3 g of asbestos in 7 ml of 10 % hexachloroplatinic (IV) acid, evaporating the mixture to dryness, and igniting the residue at red heat.
A 250-ml distilling flask with a short side-arm at least 10mm wide. The flask is fitted with a gas inlet tube reaching just above the surface of 100g of pure iodine contained in the vessel. The flask is connected with a glass tube, 80-100 cm in length and 2 cm in diameter, which is packed with platinized asbestos for a distance of about 15 cm, starting 5 cm from the flask containing the iodine.
A circular oven is arranged to heat only the forward portion of the tube. The outlet connected to a U-tube packed with glass or porcelain chips covered with a paste of moist red phosphorus and a dry wash bottle to prevent suck-back. Finally, the absorption vessel containing 50 ml of water which is cooled in an ice-salt bath is attached.
The air in the apparatus is displaced by a stream of dry nitrogen for 30 minutes; because if any oxygen is present when the hydrogen is introduced next, an explosion may result on contact with the catalyst. Dry hydrogen is now introduced for about one-half hour in place of the nitrogen at a rate of about 5 bubbles per second. The circular oven is slowly heated to 500° C and the flask containing the iodine is immersed in an oil bath at 160°C.
Only a slight purple color, due to uncombined iodine, should be visible in the cold part of the reaction tube; a series of loose asbestos plugs will serve to retain some of the iodine that condenses there. The blockage of the side-arm of the distilling flask by sublimed iodine may be prevented by gentle heating.
Under the conditions described, about 3 hours will be needed to convert all the iodine; approximately 70 ml of 57-59% hydriodic acid is formed in the absorption flask. Yield 80%. If 135 g of iodine are used instead in the preceding experiment, about 75 ml of 65-68% hydroiodic acid are produced.
In a distilling flask fitted with a funnel are placed 100 g of iodine and 10 ml of water. A slurry of 5 g of red phosphorus and 10 ml of water is placed in the alter funnel. A glass rod fitting snugly into the neck of the funnel serves as a valve for the periodic introduction of material into the evolution flask. The outlet tube connected to a U-tube filled with glass beads or porcelain chips coated with moist red phosphorus.
Finally two wash bottles in series filled with 50 ml. Both bottles are surrounded by an ice-salt bath. In order to avoid a suck-back of the water in the absorption flasks, it is advisable to place an empty safety trap between the U-tube and the water traps. The reaction flask is cooled in water while the phosphorus slurry is slowly added, dropwise, to the iodine; evolution of gas proceeds smoothly.
Great care must be taken to add the aqueous slurry of red phosphorus slowly, especially at the beginning of the reaction; otherwise the gas may be evolved with explosive violence. When all the phosphorus has been added, the flask is gently warmed to complete the reaction. If the residue in the flask is still iodine-colored, a little more phosphorus may be added. Yield of hydroiodic acid is 90 % based on the iodine.
Two hundred and eighty-five grams of iodine are suspended in 250 ml of water contained in a one-liter filter flask fitted with a wide gas inlet tube that reaches to about one-half inch from the bottom of the vessel. Under the hood, a stream of hydrogen sulfide is passed into the suspension as rapidly as it can be absorbed. Some heat is evolved and the flask may be cooled in a water bath.
Hydroiodic Acid: Frequent agitation is necessary to prevent a coating of sulfur from forming over the initially undissolved iodine; as the reaction progresses, the iodine dissolves in the hydriodic acid already formed. The sulfur, which settles out as a spongy mass on the bottom of the flask, is separated from the pale brown liquid that is finally produced by decantation, and filtration through a plug of glass wool.
The filtrate is refiuxed to remove excess dissolved hydrogen sulfide, using boiling chips to prevent bumping. Five milliliters of 30% hypophosphorous acid are added, the mixture is fractionated through a short efficient column, and the portion boiling at 125-127° C is collected. Yield 425~450 g of 57 % hydroiodic acid.
Hydriodic acid that is stored in a dark all-glass container in the refrigerator, preferably under an inert gas, remains colorless for 4-3 months. If the treatment with hypophosphorus acid is omitted, the resulting acid is straw-colored and it deteriorates more rapidly on standing; only distillation in hydrogen or carbon dioxide gives a colorless material.
Hydroiodic Acid: Inorganic laboratory preparations 145-147, 1962