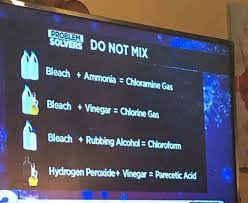
How to Make Chloroform
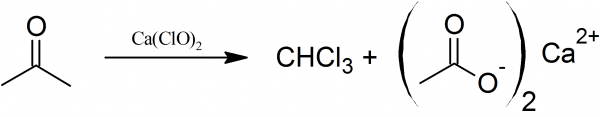
How to Make Chloroform: 200 g of fresh bleaching powder are ground up to a thin paste with 800 ml of water. The mixture is transferred to a large flask fitted with a distillation condenser and receiving flask. 40 g of acetone or ethanol are then added and the reaction mixture is cautiously heated until the reaction sets in.
How to Make Chloroform: The chloroform is removed by distillation and the distillate is washed with dilute solution of sodium hydroxide, dried over calcium chloride, and then redistilled. Chloroform is a heavy colorless liquid with a sweet smell boiling point 61° C. Yield about 40 g.
Preparation of organic compounds, E. de. Barry Barnett, 78, 1912
Preparation of chloroform is presented in the following video by Doug’s Lab.
How to Make Chloroform: A 20 % sodium chloride solution is heated at 100° C and stirred. As anode a carbon spatulas is used and 5-6 amp. current is passed. Acetone through the pipe is introduced into the bottom of the flask and is acted upon by the chlorine, forming a compound intermediate chlorinated compound which is decomposed by the action of the sodium hydroxide produced, giving chloroform. The yield of chloroform is 85 % of the theoretical.
Organic medical chemicals, by M. Barrowliff, 23, 1921
Rev. d. Prod. Chim. 3 309