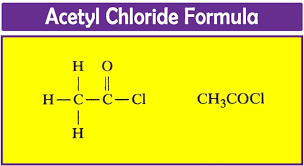
Preparation of Acetyl Chloride
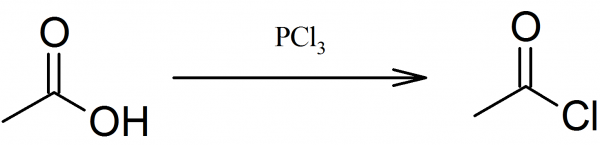
Most commonly acetyl chloride prepared by the action of phosphorus trichloride on acetic acid, since acetyl chloride boils at 51° C and it is easily separated by simple distillation from the nonvolatile phosphorous acid. Phosphorus pentachloride reacts equally well, but in this case, the second reaction product is phosphorus oxychloride, which boils at 107° C and the separation is not easily accomplished.
However, acetl chloride prepared from phosphorus oxychloride is very pure with very constant boiling and does not become turbid on standing. Preparation of acetl chloride with commonly used thionyl chloride is not practical as it cannot be removed effectively from the reaction product.
To 100 g of glacial acetic acid in round bottom flask connected with a condenser, 80 g of phosphorus trichloride are added through a dropping funnel. Hydrochloric acid gas appears and the reaction rate is regulated by cooling the reaction mixture with cold water. After all phosphorus trichloride has been added the reaction flask is gently heated to 40-50° C and the liquid which was homogeneous before heating separates into two layers.
To separate acetyl chloride which forms the upper, lighter layer, from the heavier layer of phosphorous acid, the mixture is heated until nothing more passes over. Since acetyl chloride is very easily decomposed by moisture, the distillate must be protected from the air with a calcium chloride tube. For complete purification of crude acetyl chloride, the distillate is redistilled. The portion distilling from 50-56 °C is collected in a separate vessel. Boiling-point of pure acetyl chloride, 51° C. The yield of acetyl chloride is 80-90 g based on glacial acetic acid.
For the preparation of acetyl chloride, sodium acetate should be prior dried. In order to dehydrate sodium acetate, it is placed in a shallow iron or nickel dish and heated over a free flame. Sodium acetate first melts and on further heating the mass solidifies. In order to remove the last portions of the water, the salt is heated further with a large flame, until the solidified mass melts for the second time. In a 5 liter flask, 1300 g of freshly fused sodium acetate are placed.
The flask is equipped with a reflux condenser and a separatory funnel through which 1300 g of phosphorus oxychloride are added. After it has been allowed to run in slowly, the reaction mixture is left for 10-12 hours. After this period, the flask is equipped with a condenser set for downward distillation and a receiver which should be either a boiling or suction flask fitted tightly to the condenser and holding a calcium chloride tube in the side arm.
The mixture is then heated, the acetyl chloride distills slowly so that it is necessary to heat for 12-15 hours, or even longer. The yields vary between 630 and 750 g of pure acetyl chloride boiling at 49-52° C. The acetyl chloride as it is formed is so pure that by using a fractionating column on the original reaction mixture flask, pure acetyl chloride may be distilled directly and no redistillation is required.
Organic Chemical Reagents, by A. Roger, 77-79, 1919