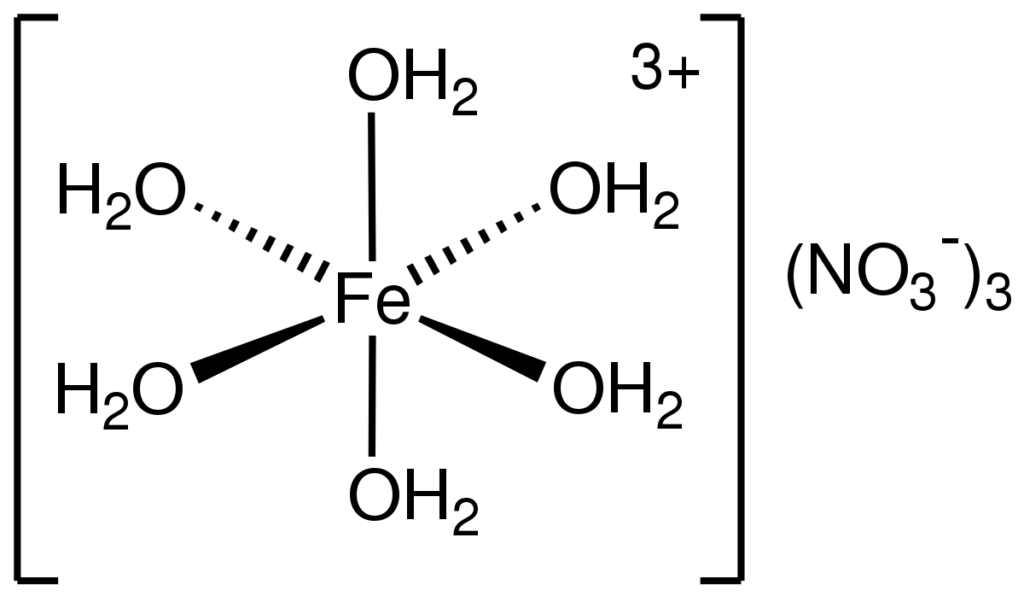
Preparation of Iron iii Nitrate

Iron iii Nitrate: To a mixture of 100 ml of concentrated nitric acid and 30 ml of water, 50 g of iron filings or fine turnings in small portions are added. The rate of reaction can be regulated by the rate of addition of iron and by gently heating the mixture but not above 70° C. When the iron has practically all dissolved, the solution is filtered and set it aside to crystallize.
The filtrate is usually dark in color owing to the presence of colloidal basic nitrates. These salts are gradually converted into the normal nitrate, and the color becomes very much lighter. If no crystals form after standing the crystallization can be initiated by addition of concentrated nitric acid since the crystals are somewhat less soluble in nitric acid than in water. The crystals of iron (III) nitrate filtered and dried in vacuum.
Laboratory methods of inorganic chemistry by H. Biltz, 1928