Preparation of p-benzoquinone (benzoquinone; quinone)
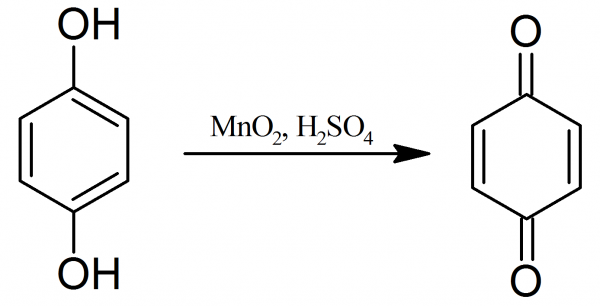
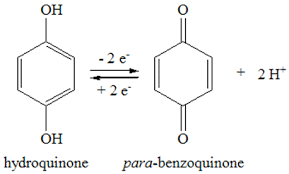
Benzoquinone: Quinone usually is prepared by the oxidation of aniline in acid solution with sodium dichromate. On a laboratory scale the yield is only about 35% and the process is somewhat lengthy. A rapid method of preparing pure quinone consists in oxidizing hydroquinone with manganese dioxide in an acid medium and removing the product from the reaction mixture by steam distillation. Oxidation of hydroquinone is a more satisfactory regarding starting material than aniline for the laboratory production of quinone. Furthermore, the yield is much better.
The reaction is carried out in a 2-liter flask having a long neck or an adapter suitable for steam distillation and equipped with the following special condensing system to prevent the loss of quinone, for this substance is very volatile with steam and sublimes easily.
A long water condenser and fitted the lower end of the condenser tube, connected by means of an adapter, to a 2-liter round-bottomed, short-necked flask submerged as far as possible in a large tub of ice and water. Through a second hole in the stopper of this receiving flask the end of a second condenser clamped in a vertical position is inserted.
The main condensation takes place in the receiving flask. The whole apparatus must be ready for immediate use, with the cooling water running and the ice in place, before mixing the reagents. In the reaction flask, 240 ml of water and then 45 ml of concentrated sulfuric acid, followed by 55 grams of hydroquinone are placed. To the obtained mixture 70 grams of powdered manganese dioxide are added.
The contents are mixed and at once the flask is connected with the receiving system and steam distil. The oxidation is a brisk reaction and the quinone distils so rapidly at first that it may clog the tube of the first condenser. To prevent of this, the run of water is stopped in the condenser until the yellow solid is all melted. The distillation is finished in 30-60 minutes.
Any material lodged in the two condensers are removed into the receiver with a long rod. The distillate is thoroughly cooled and the product is collected. A loss of weight due to sublimation is minimized by drying for a short time. The final yield of p-benzoquinone is 35-40 grams, furthermore, and the product usually requires no further purification. Pure p-benzoquinone melts at 116° C and darkens when exposed to the light.
Experiments in Organic Chemistry, L. F. Fieser, 228-229, 1941