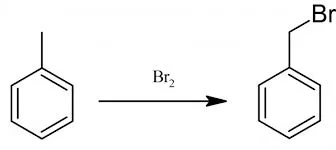
Preparation of benzyl bromide
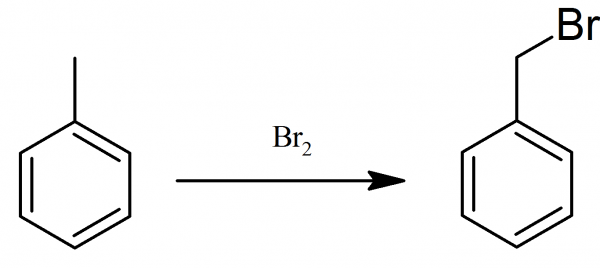
Benzyl bromide, as in case with benzyl chloride, is prepared by action of bromine on toluene.
To the perfectly dry round bottom flask fitted with a reflux condenser, stirrer and dropping funnel 50 g toluene are placed. As the reaction flask is exposed to direct sunlight (or 500W photolamp) 75 g elemental bromine are slowly added with vigorous stirring.
As the reaction proceeds the solution, which is first colored reddish-brown, becomes colorless. When all the bromine has been added, benzyl bromide is fractionally distilled and the fraction boiling between 190-205° C. collected separately. This fraction is further fractionated through an efficient Vigreux column.
The war gases chemistry and analysis, by M. Sartory, 132-133, 1939.
By slightly modified procedure benzyl bromide could be prepared from 0.2 mole of the toluene which is dissolved in a five-fold amount of dry carbon tetrachloride and is charged into a two-necked flask with a reflux condenser and a well-clamped dropping funnel.
The dropping funnel should dip into the liquid in order to limit losses of bromine. The flask is heated to the boil and for each atom of hydrogen to be replaced there is added dropwise 0.205 mole of elemental bromine (bromine should be dried by shaking with concentrated sulfuric acid).
During the bromination reaction, the mixture is irradiated with a 500-watt photolamp. The rate of addition of the bromine must be adjusted in such a way that the carbon tetrachloride dripping from the reflux condenser always remains practically colourless. The monobromination reaction takes about 30 min to 2 hours. The hydrogen bromide evolved is led through the reflux condenser, which carries a pierced stopper with a glass tube and PVC tubing, into an Erlenmeyer flask half-filled with water.
The inlet tube should not dip into this but end about 1 cm over the surface of the water. At the end of the reaction, the irradiation is stopped. The cooled solution is rapidly washed with ice-water, then with ice-cold aqueous sodium bicarbonate solution, and again with ice-water, and is dried with magnesium sulfate, and the carbon tetrachloride is evaporated off in vacuo. The residue or is distilled in vacuo (b.p. 78°C/15mm) after the addition of a spatulatip of sodium bicarbonate yielding 70% of benzyl bromide.
Organicum. Practical Handbook of Organic Chemistry, by Heinz Becker, Werner Berger and Günter Domschke, Addison-Wesley Pub. Co, 172-174, (1973)