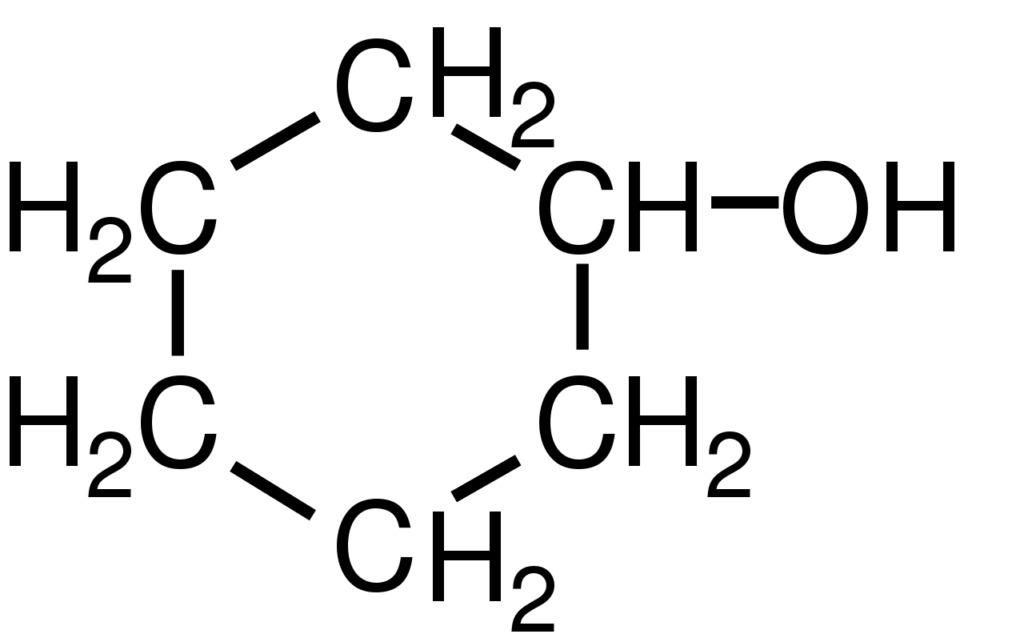
Preparation of Cyclohexanol
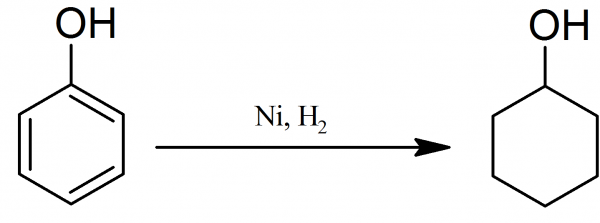
Cyclohexanol: For the preparation of nickel catalyst, small pieces of pumice stone of a convenient size are soaked in a concentrated solution of nickel nitrate in distilled water, and heated in a basin over a free flame until the nitrate has been converted into the nickel oxide. Cyclohexanol: Alternatively, the pumice is impregnated with a paste of its own weight of nickel oxide and distilled water, and dried on a water bath. The nickel oxide is reduced by heating in a current of pure, thoroughly dried hydrogen in a combustion tube.
A combustion tube 1 metre long is loosely packed for three-quarters of its length with the impregnated pumice stone, the layer being held in position with loose asbestos plugs. The tube is placed in a long cylindrical air bath fitted with 2 thermometers. The hydrogen for the preparation of catalyst should be passed first through sodium hydroxide solution, then through conc. sulphuric acid, then over heated copper gauze or turnings (previously washed with alcohol to remove grease), and finally through a tower containing sticks of sodium hydroxide.
Cyclohexanol: Ordinary corks should be used, and not rubber stoppers, in making the connections to the combustion tube. Unpurified hydrogen must not be admitted, as otherwise the catalyst will be poisoned. It is convenient to have a by-pass in the form of a T-piece between the copper gauze and the sodium hydroxide tower.
At first the hydrogen escapes through the by-pass. After the air has been expelled (a sample must be collected and tested) as far as the T-piece, the copper gauze tube is heated, and the current of hydrogen is then diverted through the tower into the combustion tube.
Some should also be allowed to escape through the funnel B to remove air from its stem. When the air has been expelled completely, the air bath carrying the combustion tube is heated to 280° C. Air must not be allowed to enter the combustion tube from this until the end of the experiment. The reduction of the nickel oxide will take at least a week; the hydrogen is passed at the rate of about 300ml per minute.
The reduction is accompanied by a colour change from black to a greyish-yellow, and is complete when no more steam is evolved, i.e., when a calcium chloride tube at the exit end of the combustion tube does not gain in weight after passing the exit gas through it for ¼ hour.
The apparatus is as in synthesis of cyclohexane except that in front of the catalyst tube is inserted a small distilling flask weighed before and after the experiment containing 100 g of pure redistilled phenol.
Hydrogen enters by means of a one-holed cork in the neck of the flask and leaves by the side tube, which fis into a one-holed cork at A. During the reduction f the nickel oxide the tube by which the hydrogen enters the flask is raised a little above the surface of the phenol.
After reduction is complete and the temperature of the catalyst has been reduced to 180-190° C, the phenol in the flask is heated almost to its boiling point, and the hydrogen delivery tube pushed well down into the liquid. Care must be taken that phenol does not condense in the tube, and that only the vapour passes over.
Cyclohexanol: When sufficient liquid has condensed in the receiver, it is shaken with sodium hydroxide solution to remove unchanged phenol, extracted with ether, and the extract dried over anhydrous potassium carbonate for 24 hours.
Cyclohexanol: The ether is removed on a water bath, and the residue distilled; the fraction 156-164° C is collected and refractionated between 159-161° C. Yield almost theoretical (10.5 g of cyclohexanol per 10 g of phenol). Colourless liquid; aromatic smell, insoluble in water; b.p. 161 °.