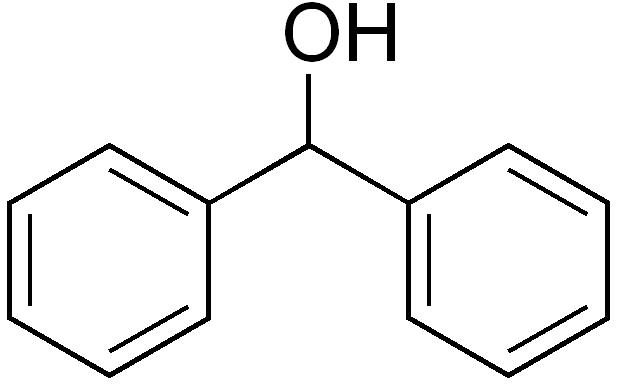
Preparation of Diphenylmethanol
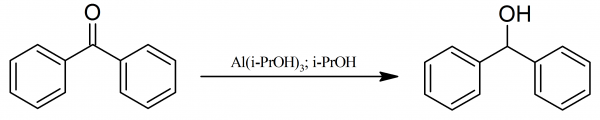
Diphenylmethanol: The reaction flask is attached to a short reflux condenser through which, however, no water flows, instead is filled with methanol. The upper end of the reflux condenser is fitted to a small, water-cooled, descending condenser and a receiver. The reaction flask is charged with 18.2 g of benzophenone and a solution of 20 g of aluminum isopropoxide dissolved in 100 ml of dry 2-propanol.
The reaction mixture is heated on a water-bath so that 5-10 drops pass over per minute. After 30-60 min no more acetone is detectable in a sample of the distillate. If more than 50 ml or 60 ml of 2-propanol distils over, fresh 2-propanol must be added to maintain the volume. Water is then passed through the condenser and the mixture is heated to vigorous refluxing for 5 min.
The water-cooling is then discontinued and the first five drops distilling are tested for acetone with 2,4-dinitrophenylhydrazine in hydrochloric acid; if the test is positive, distillation is continued until it becomes negative. Then most of the 2-propanol is removed under slightly reduced pressure. The cold residue is hydrolyzed with dilute hydrochloric acid prepared from 35 ml of concentrated hydrochloric acid and 175 ml of water, and the aqueous suspension is shaken until hydrolysis is complete.
The crystalline diphenylmethanol is filtered and washed with cold dilute hydrochloric acid and then with water yielding 18.4 g of crude reaction product. Diphenylmethanol is purified by crystallization from hot light petroleum (b.p. 60-70° C) and if the solution is not clear, benzene is added and the hot solution is filtered. The diphenylmethanol crystallizes, on cooling, as colorless needles with melting point 67-69° C. Yield is 18.2 g or 99%.
Benzopinacol is produced during the photochemical reduction, but it is at once cleaved by the sodium alcoholate; the benzophenone formed by cleavage is converted into more benzopinacol, cleaved and eventually consumed.
In a round-bottomed flask, 10 grams of benzophenone are dissolved in 60-70 ml of isopropyl alcohol by warming. Then the flask is filled to the neck with more of isopropyl alcohol and very small piece of sodium (0.05 grams) is added. The flask is tightly stoppered and inverted upside down in a beaker. Then the flask is exposed to the best available sunlight. The reaction is complete when, after exposure to the sunlight, the greenish blue color disappears. The solution is diluted with water, acidified and evaporated. The diphenylmethanol is obtained directly pure, with melting point 68° C.
Experiments in Organic Chemistry, L. F. Fieser, 104, 1941
JACS, 55, 391 (1933).
10 g of benzophenone are dissolved in 200 ml of alcohol, and 40 g of 50% aqueous potassium hydroxide added, and the mixture boiled until it turns dark brown. The mixture is treated gradually with 100 g of zinc dust until the brown colour disappears. The hot solution is filtered, and the filtrate poured into ice-water acidified with hydrochloric acid. Benzhydrol separates and may be crystallised from alcohol. Yield 90%, colourless needles; slightly soluble in water, m.p. 68° C.
Systematic organic chemistry, by W. M. Cumming, 186, 1937.