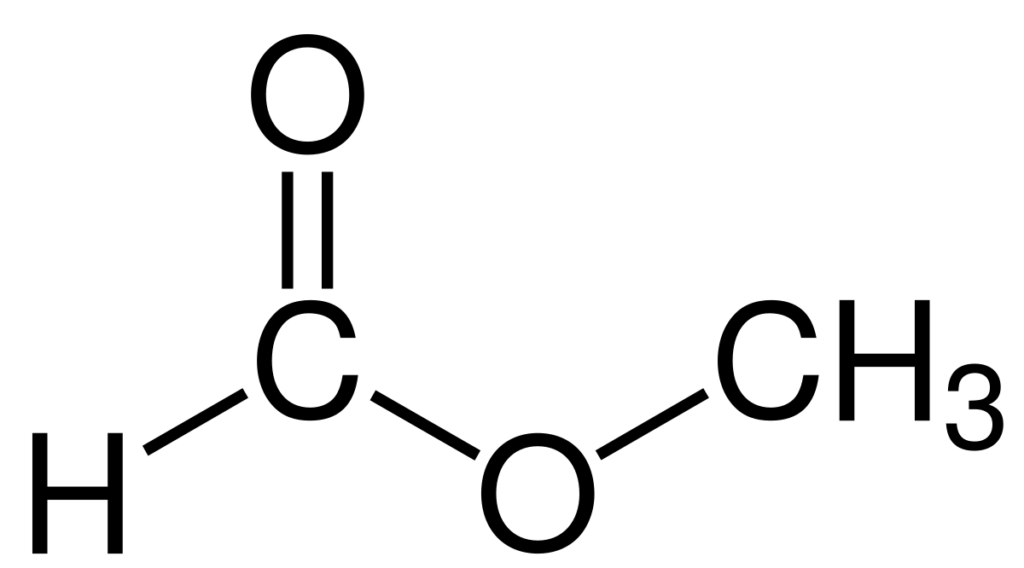
Preparation of methyl formate
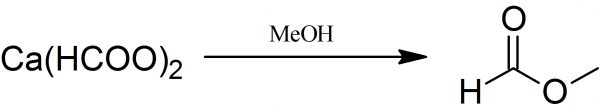
Methyl formate is prepared by the action of methyl alcohol saturated with hydrochloric acid on calcium formate.
To the round-bottom flask equipped with dropping funnel and a reflux condenser, the latter being connected with a well-cooled descending condenser 100 g of calcium formate are placed. 130 ml methyl alcohol saturated with hydrogen chloride (methanol could absorb up to 40 % of hydrogen chloride) are placed in the dropping-funnel and added to the calcium formate.
When all the methanol has been added, the mixture is allowed to stand for a short time and then methael formate is distilled. The reaction product is washed many times with saturated sodium chloride solution and neutralized with sodium carbonate. Water and methanol is separated by allowing to stand with a large quantity of fused and finely ground calcium chloride for 24 hours. The reaction product and calcium chloride form a crystalline compound, which by simple distillation leads to pure methyl formate. Methyl formate is a liquid boiling at 31-32° C.
The war gases chemistry and analysis, by M. Sartory, 100-101, 1939.