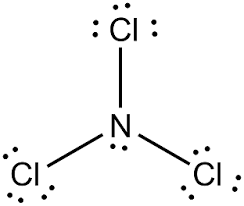
Preparation of Nitrogen Trichloride

Nitrogen Trichloride: In 1 liter round bottom flask 225 ml of chloroform with 10-20% carbon tetrachloride is placed. To this mixture 600 ml of a 10% solution of ammonium sulfate was added. The flask is plugged with stopper having inlet and outlet tubes. The outlet tube is attached to a flask containing an excess of alkali to absorb chlorine.
To control the reaction the flask should be cooled with ice-water. Then, the inlet tube is attached to the chlorine generator and strong current of chlorine while shaking the reaction mixture is passed into the solution for an hour.
Then two layers were separated and the chloroform layer was washed with a 5% solution ammonium sulfate, thoroughly shaking the mixture of these solutions for 5 min. At the end the layers are carefully separated from each other and dried with calcium chloride. The solution contains about 12% of nitrogan trichloride.
Solution of nitrogen trichloride when stored in the dark under ammonium sulfate solution is stable for several days. Before use it again after thorough shaking nitrogen trichloride is separated from the (ammonium sulfate solution and dried as indicated. Working with such solutions is not dangerous as long as the content of nitrogen trichloride does not exceed 18%.
Руководство по препаративной неорганической химии, Г. Брауэра, 238-239, 1956 (Guide to preparative inorganic chemistry, H. Brauer, 238-239, 1956 )