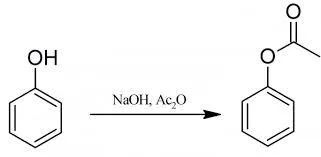
Preparation of Phenyl Acetate
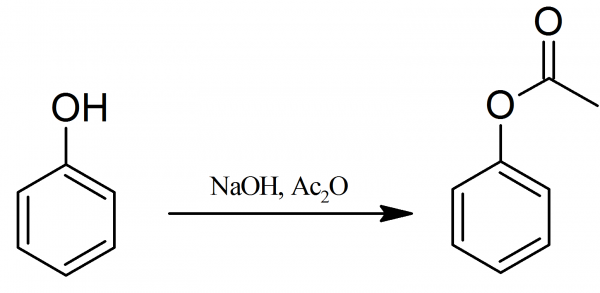
Phenyl Acetate: 15 g of phenol are dissolved in 105 ml (1.6 moles) of 10% aqueous sodium hydroxide solution, and the solution is cooled by adding about 150 g of crushed ice. Then 22 ml (or 24 g) of acetic anhydride is added, the flask is stoppered and is shaken vigorously for about 5 minutes. When the emulsion of phenyl acetate is obtained the reaction is complete. The mixture is poured into a separating-funnel, owing to the density of the acetate being only slightly greater than that of water, a sharp separation is usually not rapidly obtained.
To overcome this obstacle 8 ml of carbon tetrachloride is added to the separating-funnel, and after shaking, a sharp and rapid separation of the heavy solution of the phenyl acetate in the carbon tetrachloride is obtained. The upper aqueous layer is discarded and phenel acetate solution treated again in the separating-funnel with about 80 ml of very dilute sodium carbonate solution.
Phenyl acetate is separated and dried with calcium chloride for 20-30 minutes and then filter directly into a 50 ml distilling-flask. The distillation flask containing crude phenel acetate is fitted with a 360° C thermometer and an air-condenser. The distillation is performed slowly by heating over a gauze. The boiling-point of the mixture rises slowly but steadily to about 170° C before the distillation of the carbon tetrachloride is complete, and then rises rapidly to about 193° C.
The fraction passing around 190-200° C is collected yielding about 20 g of pure phenyl acetate as a colorless liquid, with boiling point 196° C and d=1.08, almost insoluble in water and almost odorless.