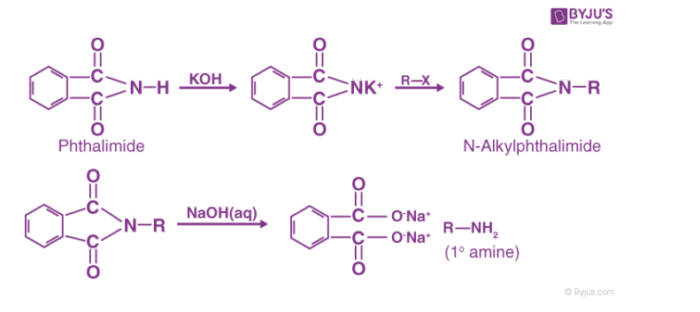
Preparation of phthalimide
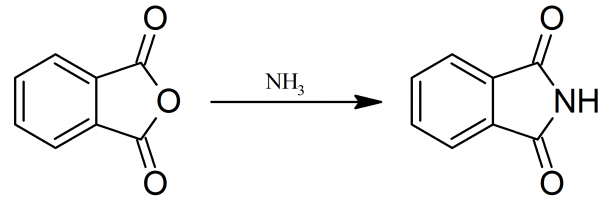
phthalimide: In a three-necked flask fitted with thermometer, gas introduction tube, and wide outlet tube bent downward, 148 grams (1.0 mole) of pure phthalic anhydride is melted and heated to about 170°. At this temperature a rapid stream of ammonia is passed into the molten phthalic anhydride. The ammonia is completely absorbed and steam escapes, carrying with it some phthalic anhydride.
Sublimed material which condenses in the neck of the flask is melted down from time to time. During the introduction of the ammonia, the temperature is raised slowly until it reaches 240°. At this point, the phthalimide does not solidify since its melting point is 230°. When ammonia begins to escape, the reaction is nearly complete.
The stream of ammonia is continued for 10 minutes more, and the melt is immediately poured into a porcelain dish where it is allowed to solidify. About 130 to 135 grams of phthalimide is obtained, melting at 223° and containing as an impurity only traces of unchanged phthalic anhydride.
The fundamental processes of dye chemistry, by H. E. Fierz-David, 173-174, 1949