Preparation of Potassium Ethyl Sulfate
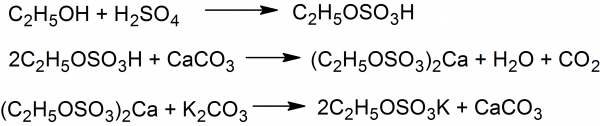
Ethyl Sulfate: To 80 ml of ethyl alcohol 20 ml of concentrated sulfuric acid are slowly added and well mixed by shaking. A considerable amount of heat is developed in the process. The reaction flask is heated for 2-3 hours under reflux, yielding ethyl hydrogen sulfate and small amount of sulfuric acid, unchanged ethyl alcohol. The reaction mixture on cooling with well stirring is poured into 500 ml of cold water and neutralized by adding calcium carbonate.
Addition of calcium carbonate converts the free sulfuric acid to calcium sulfate and the ethyl hydrogen sulfate into the soluble calcium salt of ethail sulfate. The neutralized mixture is gently heated, then filtered, the calcium sulfate is washed with a little of water. To obtain potassium ethyl sulfate the clear filtrate is heated and a solution containing about 50 g of potassium carbonate is added in small quantities until the liquid is highly alkaline.
The precipitated calcium sulfate is removed by filtration, and the filtrate evaporated to dryness yielding crude potassium ethyl sulfate, which contains some impurities (calcium sulfate, calcium carbonate, potassium carbonate). To obtain pure product, crude potassium ethyl sulfate is purified by recrystallization from methanol.
A class-book of organic chemistry, by J. B. Cohen, 31-32, 1918

