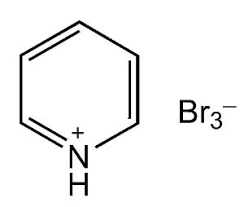
Preparation of Pyridinium Tribromide
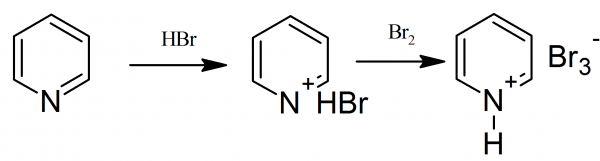
Pyridinium Tribromide: To the 1 liter round bottom flask equipped with a reflux condenser and a dropping funnel 79.1 g of pyridine were placed, and while cooling the flask with ice-water, slowly from a dropping funnel 113 ml of 48% hydrobromic acid was added. Then, the reflux condenser is removed and replaced with distillation head. Water was distilled under vacuum by heating the flask in an oil or glycerol bath to 160 ° C.
Pyridinium Tribromide: After distillation the flask should remain completely dry with pyridine hydrobromide, which in the same flask were completely dissolved by heating with 240 g of glacial acetic acid. The mechanical stirrer is inserted to the middle neck of the flask and to reaction mixture, with stirring, at a temperature of 60-65 °, slowly from a dropping funnel a solution of 80 g (25.5 ml) of bromine in 80 ml of glacial acetic acid were added.
Pyridinium Tribromide: The flask content were transferred to a 1 liter beaker and covered with glass, allowed to crystallize. On cooling from the solution red-brown needles of pyridinium tribromide form. After cooling to 10° C the separated crystals are filtered on a Buchner funnel, washed and dried in air. The yield is about 190 g of product with melting point 101-104 ° C.
Препаративная органическая химия, Вульфсон Н.С., 202-203, 1959 (Preparative organic chemistry, Wolfson N. S., 202-203, 1959);
J . Amer. Chem. Soc, 70, 417 (1948);
J. Chem. Soc, 2670, (1952);
By another method pure pyridine is treated with an excess of hydrochloric acid and saturated with bromine. A dark brown oil is formed. The aqueous layer is removed. The oil rapidly crystallizes when kept in a freezing mixture; and the oil sucked off from these crystals gives a second crop when cooled again. The solid products are washed with chloroform and dissolved in alcohol at 40-50° C, from which they crystallize on cooling.
Ber. Deut. Chem. Ges., 56, 1262 (1923);
J . Amer. Chem. Soc, 51, 863 (1929);
Pyridinium tribromide could also be prepared from pyridine hydrobromide: 1 mole of bromine in 160 g of glacial acetic acid are added to a solution of 1 mole of pyridine hydrobromide dissolved in 240 g of glacial acetic acid with stirring at about 60° C. On slow cooling of this mixture, red needles or prisms (about 300 g; m.p. 132-134° C) crystallize; they are collected under suction and dried in a vacuum-desiccator.
J. Amer. Chem. Soc, 51, 865 (1929);
It is possible simply the procedure by adding 1 mole of bromine to 1 mole of pyridine in 2 moles 48 % hydrobromic acid.
J . Amer. Chem. Soc, 70, 417 (1948).
J. Chem. Soc, 2670, (1952);