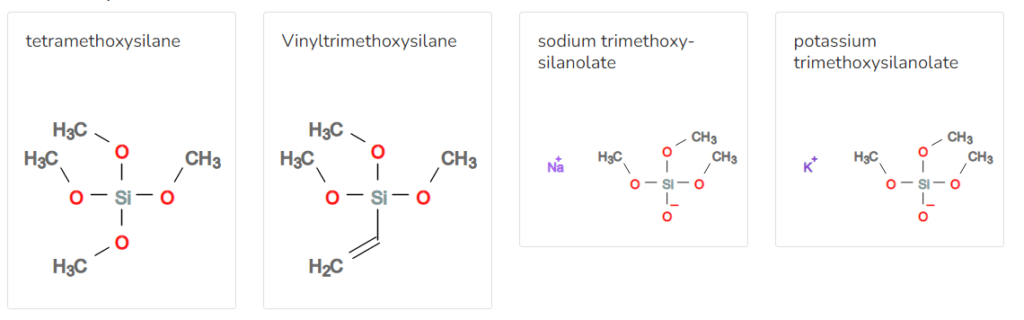
Synthesis of Si OCH3 4
1642 gm of crude product prepared in Example 3 was added to the distilling flask and heated slowly. Distillation fractions were collected at the head temperatures shown in Table 4 . From the composition of the fractions, the 1642 gm of crude product afforded 660 gm trimethoxysilane, which is more than that calculable from the composition of the starting material (first row of Table 4).
The additional tri-methoxy silane was formed via the disproportionation of the mixed dimethylaminomethoxysilanes,. The vapor phase methanolysis consumed 2521 gm tris(dimethylamino)silane and 1005 gm methanol and produced a total of 1166 gm trimethoxysilane. The stoichiometric amount of trimethoxysilane obtainable from 1005 gm (31.4 moles) methanol is 1277 gm.
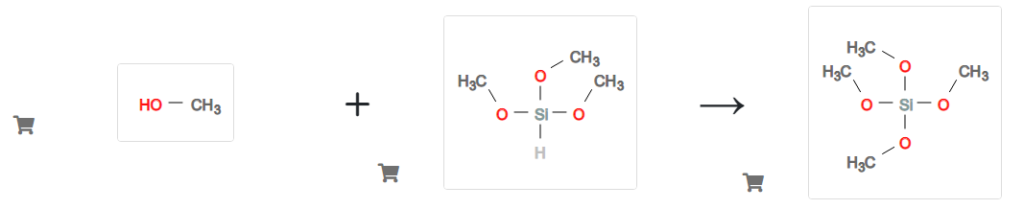
So the yield of trimethoxysilane was 91.3 wt. % based on methanol converted. Note, however, that the methanol not converted to trimethoxysilane in this pass through the reactor was converted mainly to the mixed dimethylaminomethoxysilanes, e.g., HSi[N(CH3)2 ]2 (OCH3), and mixed methoxydimethylcarbamatosilanes, e.g., HSi[OOCN(CH3)2 ]x (OCH3)3-x (x=1,2,3), which are recyclable. Only negligible quantities of Si(OCH3)4 were formed.
Literature source US04730074
Similar syntheses