Synthesis of Sulfur Trioxide
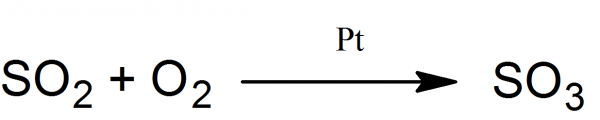
Preparation of Sulfur Trioxide
Sulfur trioxide is formed by reacting sulfur dioxide with oxygen in the present of platinum catalyst. 40 cm long, hard melted glass tube is connected to the ice-cooled receiver. In the tube 10 cm platinized asbestos is placed. Platinized asbestos could be prepared by treating asbestos with the 5 ml of 10% chloroplatinic acid solution and then drying and applying red heat. Heated chloroplatinic acid decomposes and the fibers of asbestos is covered by platinum (catalyst).
Platinized asbestos after each use should be well washed with water and heated in the red heat. Reaction equipment must be completely dry. Tube joints made from asbestos or other inert material because sulfur trioxide erodes organic materials.
Oxygen is obtained from the gas cylinder and sulfur dioxide obtained by heating the flask with 100 g of copper turnings and 400 g of concentrated sulfuric acid or by reacting sulfuric acid with sodium sulfite or by burning elemental sulfur in oxygen stream.
Oxygen and sulfur dioxide are dried by passing through sulfuric acid. The area of glass tube reactor with platinized asbestos is started to heat to a temperature of 400° C. Once the platinized catalyst temperature reaches 400° C, dry sulfur dioxide, and oxygen is introduced.
Oxygen is required a little more than the sulfur dioxide. The experiment takes up to 3 hours and during this time in the cooled collector liquid or crystalline sulfur trioxide modification condenses. Sulfur trioxide is stored in sealed glass ampoule.
Chemical lecture experiments, by F. G. Benedict, 159, 1916.
Preparation of sulfur trioxide is presented in the following video by plasticraincoat1.
Fuming sulfuric acid, consisting, as it does, essentially of a solution of ST in sulfuric acid, readily liberates the ST on heating. The fuming acid is placed in a 500 ml retort, whose neck is thrust into a 250 ml flask immersed in a freezing-mixture of salt and ice.
On gently heating the acid the sulfuric trioxide is given off and condenses in the receiver. Owing to the intensely corroding properties of the acid the retort must be very carefully heated to avoid danger of breakage. The danger is minimized if a sandbath is used.
Chemical lecture experiments, by F. G. Benedict, 160, 1916.
Phosphorus pentoxide abstracts water from sulfuric acid, setting free sulfuric anhydride. A strong sulfuric acid is placed in a distillation flask and sufficient phosphorus pentoxide is added to make a thin paste. On gently heating the mixture a vigorous reaction takes place, clouds of sulfur trioxide being given off.
Chemical lecture experiments, by F. G. Benedict, 160, 1916.
Potassium pyrosulfate, when heated, forms potassium sulfate and ST . Potassium pyrosulfate is placed in distillation flask and heated with gas burner. The sulfur trioxide is cooled and condenses in the receiver.
Chemical lecture experiments, by F. G. Benedict, 161, 1916.
Preparation of sulfur trioxide is presented in the following video by mabakken.
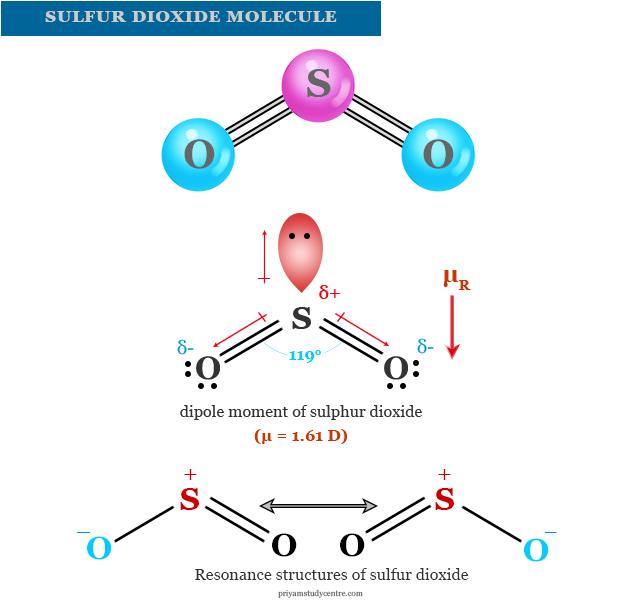
Sulfur trioxide is smoky, colorless liquid with the melting point to 16.8° C and boiling point 44.8° C. Liquid modification sulfur trioxide is unstable. Slowly it turns into a solid form. Sulfur trioxide aggressively attacks organic matter.