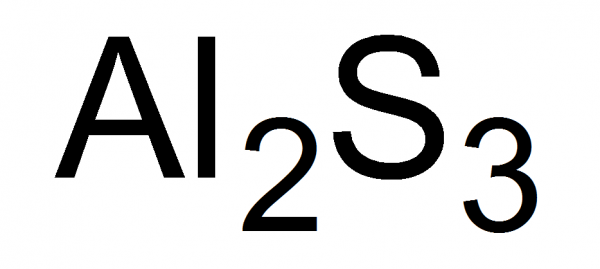Preparation of Aluminum Sulfide

Aluminum sulfide can be prepared directly from aluminum and sulfur, but when the finely divided substances are mixed and the mixture is heated, either the sulfur entirely distils off without any reaction taking place, or if a reaction starts it is too violent to control. Lead sulfide could be as a source of the sulfide ion because this substance is not volatile and cannot escape before it reacts, furthermore, the reaction is not too violent.
359 g of powdered lead sulfide and 27 g of granulated aluminum are mixed together. The mixture is transferred in the clay crucible, which is covered and placed in the gas furnace. The furnace is heated as rapidly as possible and the beginning of the reaction is indicated by appearing of a white puff of smoke.
After the reaction is complete, the crucible is kept in the furnace for 30-60 seconds, then removed with the tongs and poured the liquid contents into the iron pan. When the contents have been solidified then the brittle aluminum sulfide is cracked from the lead and aluminum sulfide is placed in a stoppered bottle. Aluminum sulfide should be well protected from the moisture as it reacts with the moisture of the air producing hydrogen sulfide.
Synthetic inorganic chemistry, by A. A. Blanchard, 149-150, 1936