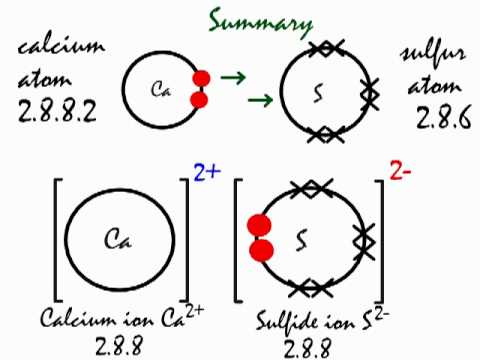
Preparation of Calcium Sulfide

Calcium Sulfide: Unless the charcoal is already very finely powdered, it should be ground thoroughly in a large porcelain mortar. 48 g of charcoal are mixed with 145 g of gypsum (CaSO4 • 1/2H2O; plaster of Paris) and placed in the clay crucible.
Calcium Sulfide: The crucible is heat in a gas furnace to between a bright red and a yellow heat for 1.5 hours. At the end of the reaction, the crucible is removed from the furnace and when cold, the contents of the crucible are inspected and there should be no unburned charcoal left. A small sample should dissolve with effervescence in hydrochloric acid and leave no residue more than a slight turbidity.
Synthetic inorganic chemistry, by A. A. Blanchard, 150-151, 1936. CS