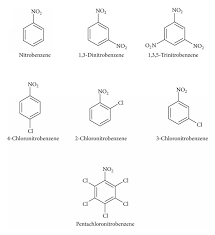
Preparation of Nitrobenzene
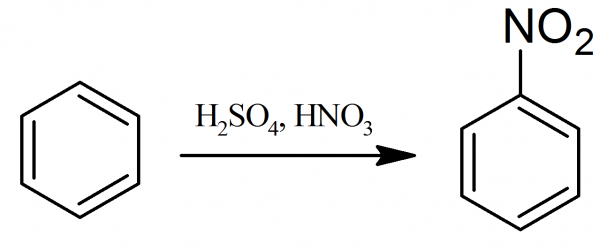
Nitrobenzene: 81.5 ml of concentrated sulfuric acid (d=1.84 g/ml) are mixed gradually with frequent shaking with 71.5 ml of concentrated nitric acid (d=1.4 g/ml). After cooling the mixture to the room temperature, by immersion in a cold water bath, gradually, with constant stirring, 57 ml of benzene are dropwise added. If the temperature rises above 50-60° C, the nitration reaction is interrupted, and the flask is cooled with the ice water.
When all of the benzene has been added, a reflux condenser is attached to the reaction flask and mixture is gently heated for an hour at 50-60° C. During the heating the flask should constantly be stirred. After cooling the reaction mixture, the lower layer, consisting of sulfuric and nitric acids, is separated from the upper layer of nitrobenzene in a separating funnel.
The nitrobenzene (d=1.2 g/ml) is then agitated in the separating funnel several times with water and dried with calcium chloride until the liquid, milky at first, becomes clear. Finally, nitrobenzene is purified by distilling and collecting fraction, which boils at 206-207 °C. The distillation process should not be performed quite to dryness or allowed the temperature to rise above 214° C. Nitration impurities such as m-dinitrobenzene and higher nitro compounds at higher temperature may decompose explosively. The final yield of nitrobenzene is 60-70 g.
Practical organic chemistry. 1956, 525-526.
9 g aniline are mixed with 50 ml water and 20 g conc. nitric acid (d=1.4 g/ml). The solution is cooled to 0-5° C and 15 g sodium nitrite in 50 ml water added. The mixture is then poured into a flask containing the copper(I) salt, prepared by dissolving 50 g copper sulphate and 15 g glucose in 100 ml water, and adding 20 g sodium hydroxide in 60 ml water to the boiling solution;
the mixture is shaken till all the copper is reduced, and is then rapidly cooled, and finally acidified by a slight excess of acetic acid. The combined mixtures are allowed to stand for 1 hour, or until the evolution of nitrogen ceases. The nitrobenzene is then separated by steam distillation. Yied 50%, yellow liquid; m.p. 5.7° C; b.p. 210° C.
Systematic organic chemistry, by W. M. Cumming, 279, 1937.