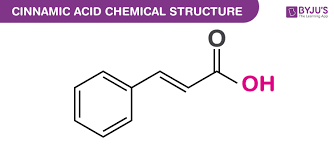
Preparation of Cinnamic Acid
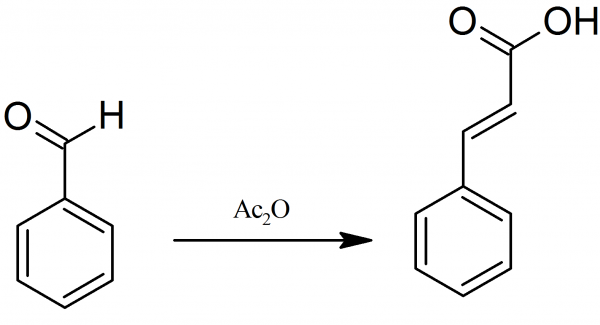
Cinnamic Acid: A mixture of 20 grams of benzaldehyde, 30 grams of acetic anhydride, both freshly distilled, and 10 grams of anhydrous pulverized sodium acetate, is heated in a flask fitted with a reflux condenser, for 8 hours, at 180° C.
After the reaction is complete, the hot reaction product is poured into a large flask. Water is added and then distilled with steam, until no more benzaldehyde passes over. The quantity of water used here is large enough so that all of the cinnamic asid dissolves except a small portion of an oily impurity.
The solution is then boiled a short time, with some activated charcoal, and filtered. On cooling, the cinnamic asid separates out in lustrous leaves. Purification is performed by crystallization from water. Reaction yield is about 15 grams and the melting point of pure cinnamic acid – 133° C.
Practical organic chemistry. 1956, 713-714.