Preparation of 1-bromobutane
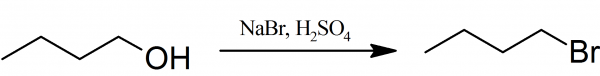
1-bromobutane: A mixture of 30 ml of water, 35 g of powdered sodium bromide and 25 ml (or 20 g) of 1-butanol are placed in a 250 ml round-bottomed flask. The flask is fitted with a dropping funnel with pressure-equalization arm. 25 ml of concentrated sulfuric acid is placed in the funnel and added dropwise into the reaction mixture with constant stirring and occasional cooling in an ice-water bath.
When the addition of sulfuric acid is complete, the dropping funnel is replaced with a reflux condenser, and the reaction mixture is gently boiled for 45 minutes, shaking the flask gently from time to time. Then the crude One-bromobutane is distilled and collected 30 ml fraction is purify by shaking with water in a separating funnel, and removing the lower layer of 1-bromobutane.
This layer is additionally treated by shaking in separating funnel with about half its volume of concentrated sulfuric acid. The lower layer of sulfuric acid is discarded and 1-bromobutane cautiously treated with dilute sodium carbonate solution, taking care to release the pressure in the funnel at frequent intervals. The lower layer of 1-bromobutane is dried with calcium chloride, filtered and finally distilled using a short column, yielding about 30 g of colorless liquid of with boiling point 99-102° C. A small residue of di-n-butyl ether, which boils at 142° C, remains in the flask.
To 250 grams of 48% hydrobromic acid contained in a 500 ml round-bottomed flask 41 ml (or 75 grams) of concentrated sulfuric acid are dropwise added. During the addition, some hydrogen bromide may be evolved. 110 ml (or 88 grams) of n-butyl alcohol are added, followed by 32.5 ml (or 60 grams) of concentrated sulfuric acid in several portions with shaking, and finally a few chips of broken glass. The reaction flask is refluxed for 2-3 hours.
During this period, the formation of 1-bromobutane is almost complete and a layer of alkyl bromide separates above the acid. When the reaction is complete the flask is cooled and arranged for downward distillation. The mixture is distilled until no more oily drops of 1-bromobutane pass over and the distillate is transferred to a separatory funnel and the lower layer containing 1-bromobutane is removed.
Crude 1-bromobutane is washed with water, an equal volume of concentrated hydrochloric acid, water, 5% bicarbonate or sodium carbonate solution, and water. The 1-bromobutane is dried with anhydrous calcium chloride or anhydrous magnesium sulfate, as completely as possible. 1-Bromobutane is purified by distillation. The fraction passing over 100-103° C is collected yielding 155 grams of pure 1-bromobutane.
The crude 1-bromobutane contains a little amount of unreacted alcohol and some n-butyl ether (b.p. 141° C). The n-butyl ether is removed by washing with conc. hydrochloric acid and this purification process is satisfactory for most purposes. Both the n-butyl alcohol and the n-butyl ether are removed by washing with of concentrated sulfuric acid; the 1-bromobutane is not affected by this reagent.
A text book of practical organic chemistry, by A. I. Vogel, 277-278, 1974
Sulfur dioxide is passed into an ice-cooled mixture of 1200g of bromine and 1300 g of crushed ice until the red color just disappears. The product is equivalent to a mixture of 15 moles of 48% hydrogen bromide and 7.5 moles of concentrated sulfuric acid. 12moles of 1-butanol are added, followed by more concentrated sulfuric acid (600 g) portion wise with stirring. The mixture is boiled under reflux for 3 hours and then distilled. The yield is 95%, and the b.p. is 101-104° C.
Org. Syn. Coll. Vol. I, 29 (1941); 26 (1956, 2nd ed).