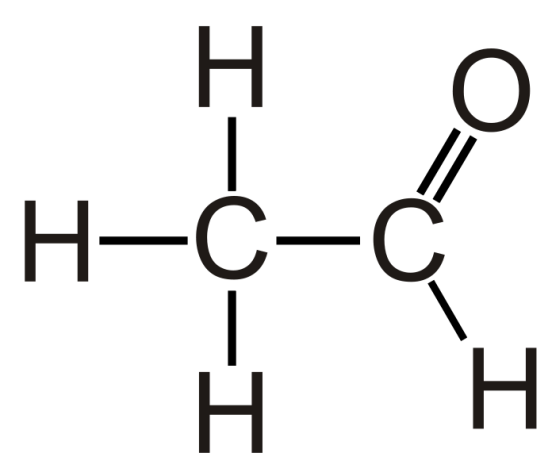
Preparation of Acetaldehyde Ethanal
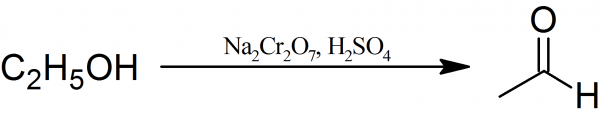
Ethanal: To a 1-liter round bottom flask fitted with a dropping funnel, a distillation head with a condenser and mechanical stirrer 60 ml of concentrated sulfuric acid dissolved in 200 ml of water are placed. The diluted sulfuric acid is heated to boiling and 200 g of sodium dichromate in 200 ml of water which has been treated with 100 g of ethyl alcohol is then added in a small portion through the dropping funnel.
During the addition of the sodium dichromate heat evolves and causes ebullition of the reaction mixture. The Ethanal, which forms during the oxidation reaction, distills into the properly cooled receiver. Besides Ethanal some side-reaction products such as ethyl alcohol, water, and acetal also distills. If uncondensed vapors of the acetaldehyde escape from the receiver, the addition of sodium dichromate is adjusted.
Also, if boiling is not caused by the addition of sodium dichromate, the reaction mixture is gently heated. Since the acetaldehyde cannot be obtained directly by fractional distillation, it is first converted into intermediate Ethanal ammonia trimer (2,4,6-trimethyl-1,3,5-triazinane), which, on proper treatment with sulfuric acid, readily yields the pure acetaldehyde.
Acetaldehyde ammonia trimer is prepared by absorbing Ethanal after distillation with ether. The ethereal solution is cooled with a freezing mixture of ice-salt, and treated with ammonia until the liquid is fully saturated. After an hour, the acetaldehyde-ammonia trimer which has separated out is collected, filtered, washed with a little ether, and dried on filter paper in a desiccator or vacuum yielding about 30 g.
In order to obtain pure acetaldehyde, 10 g of acetaldehyde-ammonia trimer are dissolved in 10 ml of water. This solution is treated with a cooled mixture of 15 g of concentrated sulfuric acid and 20 ml of water, and distilled by gentle heating collecting fraction boiling at 21° C.
For the preparation of acetaldehyde a simple fractional distillation apparatus is assembled fitted with a long all-glass Dufton column, or Hempel column. A mixture of 50 ml of paraldehyde and 0.5 ml. of concentrated sulfuric acid (as the depolymerizing agent) and a few small fragments of porous porcelain are placed in the distillation flask (the sulfuric acid may be replaced by 1-2 g of sulphamic acid (NH2SO3H) or by p-toluenesulfonic acid).
The receiver is cooled in crushed ice, meanwhile, the flask is gently heated at 50-60° C, by not allowing the temperature at the head of the column to rise above 30-32° C. The distillation must be conducted very slowly in order that the fractionation may be efficient, since acetaldehyde and paraldehyde form a constant boiling point mixture, b.p. 42° C (53.4% and 46.6% respectively).
Most of the acetaldehyde distils at 21-25° C and the distillation is stopped when 10 ml of liquid remain in the flask. The obtained acetaldehyde, produced in excellent yield, is sufficiently pure for many purposes, however, in order to obtain pure acetaldehyde, the product must be redistilled. Pure acetaldehyde boils at 21° C.
A text book of practical organic chemistry, by A. I. Vogel, 324-325, 1974