Preparation of Benzoyl Chloride
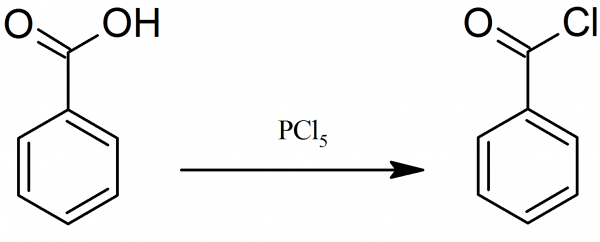
Benzoyl chloride could be prepared by reacting thionyl chloride or phosphorus pentachloride with benzoic acid.
50 grams of dry benzoic acid are treated in a 500 ml flask, with 90 grams of finely pulverized phosphorus pentachloride. The mixture is mixed well, upon which, after a short time, the reaction takes place with an energetic evolution of hydrochloric acid, and the reaction mass becomes liquid. During the reaction a lot of heat is released. After standing a short time, the completely liquid mixture is twice fractionated by collecting fraction which boils at ~ 200° C, yielding 90 % of benzoyl chloride.
Practical organic chemistry. 1956, 792-793.
A round bottom flask is fitted with a reflux condenser and a gas inlet tube. 100 grams of cold and dry benzaldehyde are added to the flask and saturated with the current of dry chlorine. The chlorine gas is readily absorbed and the evolution hydrogen chloride takes place. When the reaction has moderated, the external heat is applied by keeping gentle boiling.
The stream of chlorine is continued until no more hydrogen chloride is evolved. A stream of dry air or carbon dioxide is then passed through the apparatus in order to remove an excess of chlorine. Benzoyl chloride is obtained by distillation as a colorless fuming liquid with a very irritating smell. Yield almost quantitative.
Preparation of organic compounds, E. de. Barry Barnett, 52, 1912
19 grams of oxalyl chloride and 20 grams of the dry sodium benzoate, with 20-30 ml of dry benzene as a solvent were mixed and gently heated. 19 grams of the benzoyl chloride were obtained by fraction distillation with boiling point 198° C yielding 97 %.
Oxalyl chloride and oxalyl bromide as reagents in organic chemistry, L.H. Ulich, 26, 1918