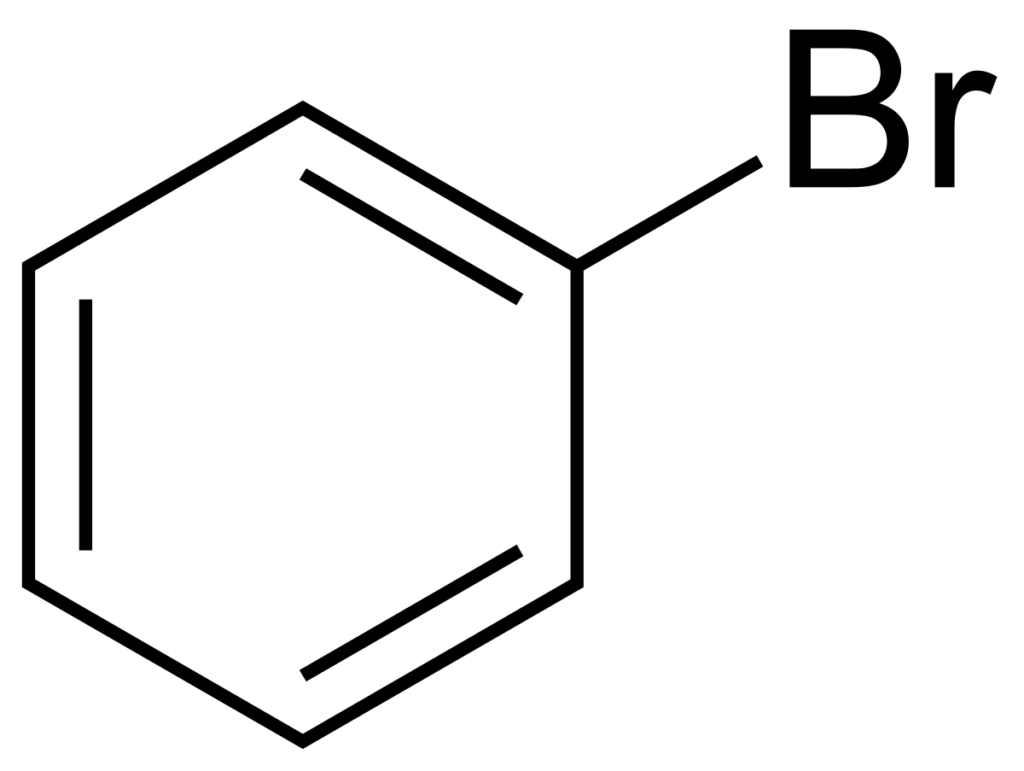
Preparation of bromobenzene
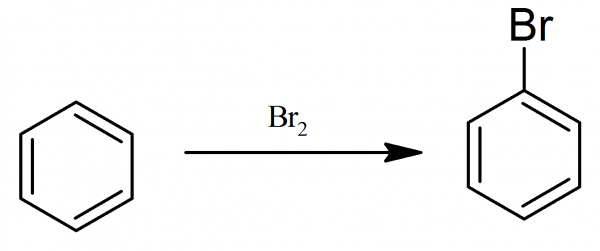
Bromobenzene: To the round bottom flask fitted with stirrer, reflux condenser and dropping funnel 50 g of dry benzene and 0.5 g of pyridine (to act as a halogen carrier) are placed. From the dropping funnel 120 g (40 ml) of bromine are slowly added.
A vigorous reaction begins at 25-30° C with evolution of hydrogen bromide. When the reaction has moderated, the temperature is slowly raised to 65-70° C and maintained at this point until the evolution of hydrogen bromide has almost ceased.
After cooling, the contents of the flask are well washed with dilute solution of sodium hydroxide, dried over calcium chloride, and then distilled. Unchanged benzene passes over at about 80-100° C and bromobenzene boils at higher temperature. Those higher boiling portions are collected and refractionated. Bromobenzene is heavy colorless oil with boiling point 155° C. Yield 60 g.
Preparation of organic compounds, E. de. Barry Barnett, 51-52, 1912
Ice is added to 130 g of concentrated sulfuric acid until the temperature reaches to 0° C. 31 g of aniline are then added, and the solution diazotized with 23 g of sodium nitrite. 120 g of potassium bromide and 40 g of copper powder are then added, and, when the reaction is over, the bromobenzene steam-distilled off. The oil which passes over is collected, washed with dilute sodium carbonate, then with water, dried over calcium chloride yielding colorless oil boiling at 155° C. Yield 42 %.
Preparation of organic compounds, E. de. Barry Barnett, 77, 1912