Preparation of Lead II Nitrate
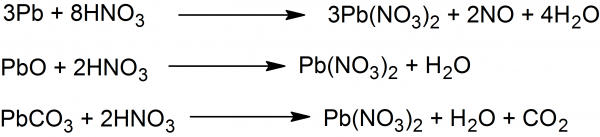
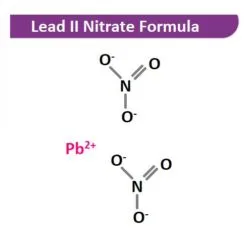
Lead II Nitrate: Lead nitrate is one of the most readily prepared salts of lead. Lead oxide (litharge), lead carbonate or metallic lead is treated with an excess amount of concentrated nitric acid. When the reaction mixture cools the crystals of lead(II) nitrate crystallizes. Lead(II) nitrate is separated by filtration and purified by recrystallization. If an insufficient amount of nitric acid is used, the basic salt of lead nitrate (Pb(OH)NO3) forms, which separates as a fine granular or flaky precipitate when the solution cooled.
Lead II Nitrate: Synthetic inorganic chemistry, by A. A. Blanchard, 277-278, 1936
Lead II Nitrate: Into an apparatus as illustrated in the following, pour into the anode compartment a salt solution prepared by adding and dissolving 200 grams (7 oz.) of sodium nitrate into 600 milliliters (20.2 fluid oz.) of water.
Lead II Nitrate: Thereafter, pour into the cathode compartment, a salt solution prepared by adding and dissolving 500 grams (17.6 oz.) of Epsom salt into 1700 milliliters (57.5 fluid oz.) of water. Thereafter, put in place the electrodes as illustrated, and then attach the power clamps; thereafter, turn on the power supply and electrolysize the cell at 6 to 12 volt at 6 amp DC for 24+ hours. After about 24+ hours, discontinue the power supply, open the cell, remove the electrodes, and then pull-out the clay pot and pour its contents into a clean beaker.
Thereafter, filter this anode liquid to remove insoluble impurities, and then recrystallize the Lead nitrate from the solution. After the recrystallization process, allow the crystals to air-dry, and then store in a plastic or glass bottle. The cathode liquid will contain allot of white precipitate of magnesium hydroxide, which can be collected by filtration if desired; after removing the insoluble magnesium hydroxide, the filtered liquid will contain dissolved sodium sulfate decahydrate, which can be collected by “low heat evaporation” if desired or simply discarded.
Kings Chem Guide Third Edition, by Jared Ledgard, 293-294, 2014.