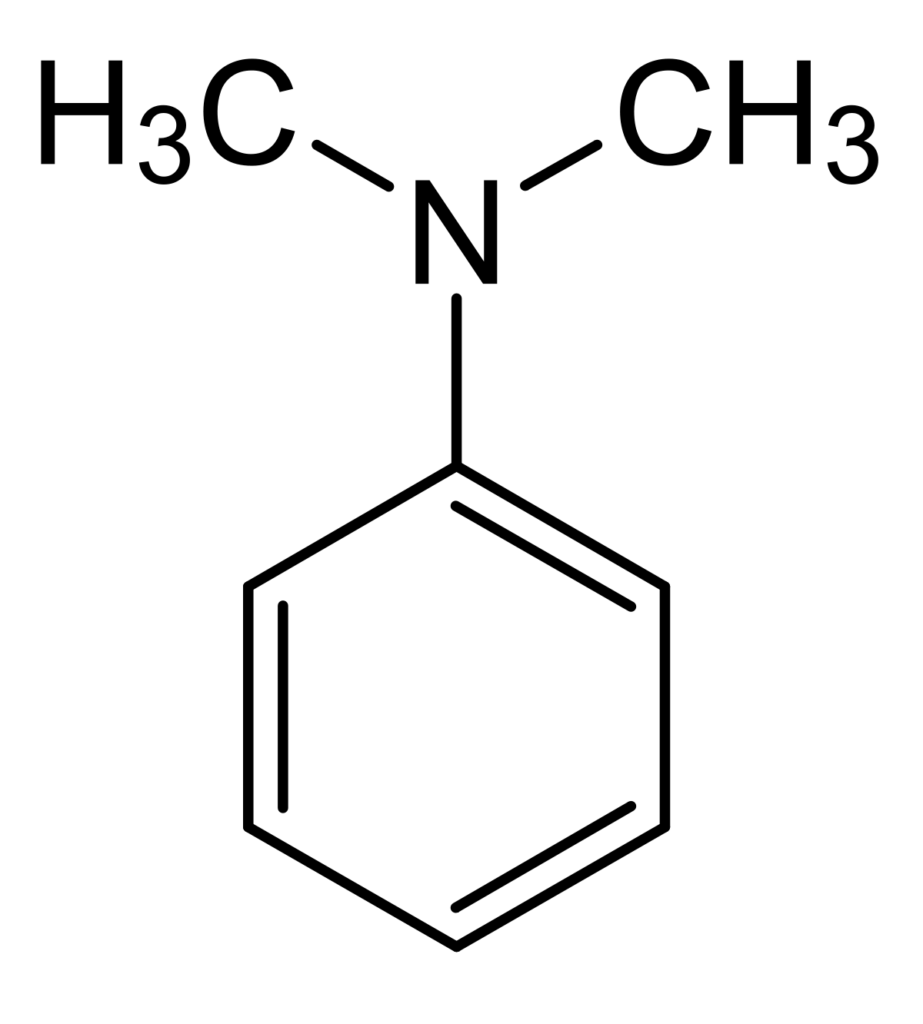
Preparation of N,N- Dimethylaniline
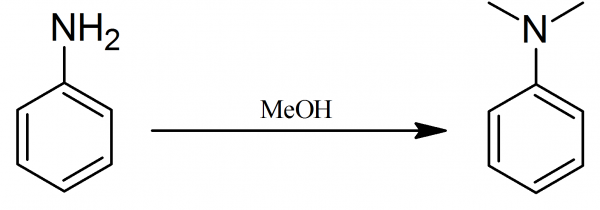
Dimethylaniline: A mixture of 75 g of aniline, 25 g of aniline hydrochloride and 75 g of methyl alcohol is heated in an autoclave for 7-8 hours at 230-240° C, the crude reaction product then made alkaline with sodium hydroxide and distilled with water steam. The distilled oil is separated with a separating funnel, dried with solid KOH and fractionated. The fraction boiling at 190-200° is collected yielding N,N-dimethylaniline with boiling point 192° C.
The Synthetic Dyestuffs and the Intermediate Products from which They are Derived, by J. C. Cain, 204-205, 1905
By slightly modified method 93 g pure aniline, 105 g pure methyl alcohol and 9-4 g conc. sulphuric acid are heated in an enamelled autoclave to 200° C. The pressure rises to about 30 atm, and the contents are left for 6 hours at 215° C. They are allowed to cool, and then 25 g 30% sodium hydroxide solution added. The product is now heated to 170° C in the autoclave for a further 5 hours.
This second heating, is necessary to decompose the sulpho-ammonium bases formed, and which are decomposed into sulphuric acid, alcohol, and tertiary amine. The contents of the autoclave, after cooling, are distilled in steam. The dimethylaniline is salted out of the distillate with common salt. It is then separated and distilled. Yield 95%, colourless liquid when pure; turns brown on standing; b.p. 192° C.
Systematic organic chemistry, by W. M. Cumming, 305, 1937.