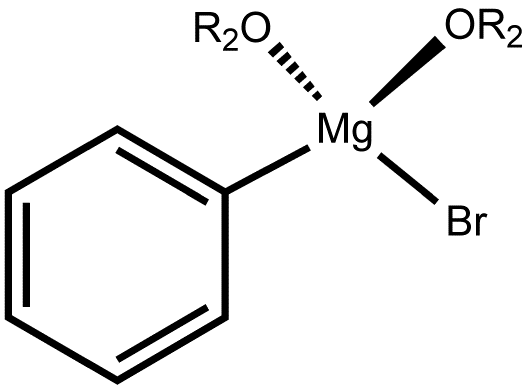
Preparation of phenylmagnesium bromide
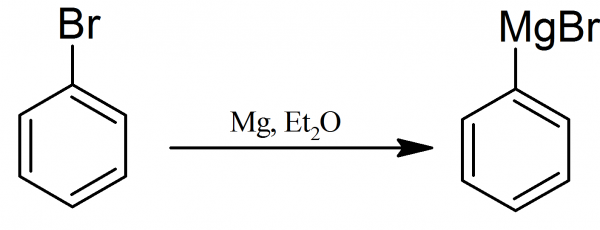
Phenylmagnesium Bromide: The formation of the Grignard reagent from bromobenzene and magnesium in ether, is difficult from the experimental point of view. For the preparation of phenylmagnesium bromide is necessary to follow the directions carefully, particularly in the matter of using completely dry reagents and dry apparatus. The provided description is used for the preparation of 0.15 mol phenylmagnesium bromide solution in ether.
The phenylmagnesium bromide is prepared in a 250 ml two-necked round-bottomed flask, fitted with an efficient reflux condenser connected with a calcium chloride drying tube and dropping funnel, which will be filled with the mixture of ether and bromobenzene. The flask, condenser and dropping funnel should be as DRY as possible.
The magnesium (3.9 grams or 0.16 mole of magnesium turnings) is placed in the two-necked round-bottomed flask, the calcium chloride tubes are attached directly to the flask, and the flask is heated gently by carefully brushing the bottom with a luminous flame.
The flask on cooling sucks in dry air through the calcium chloride. The reaction flask with magnesium is cooled to room temperature before proceeding! The calcium chloride drying tubes are removed from the flask and immediately the flask is connected with the condenser and dropping funnel (the top of the condenser and dropping funnel is connected with calcium chloride drying tube). The dropping funnel is filled with the mixture of 75 ml of dry ether and 17.7 ml (or 26.6 grams, 0.17 mole) of dry bromobenzene.
15 ml of this mixture are added dropwise to the reaction flask containing magnesium. There usually is no immediate change and it is necessary to start the reaction before adding further quantities of reagents. A very effective way of doing this is to add a small crystal of iodine and when this is done the liquid suddenly becomes slightly cloudy and ebullition commences.
When the initial reaction has been started the mixture of dry bromobenzene in ether is added dropwise at such rate that the ether boils gently from the heat of the reaction alone and without the application of external heating. This takes 25-30 minutes and when the reaction is partially complete the dropping funnel is replaced by the drying tube or stopper and the reaction mixture is gently refluxed for about one-half hour longer in order to complete the reaction.
The magnesium disintegrates and the solution acquires either a cloudy or a brownish appearance. The reaction is complete when there are only a few small remnants of unreacted metal. The solution should be used at once; it deteriorates on standing over night.
Experiments in Organic Chemistry, L. F. Fieser, 68-69, 1941