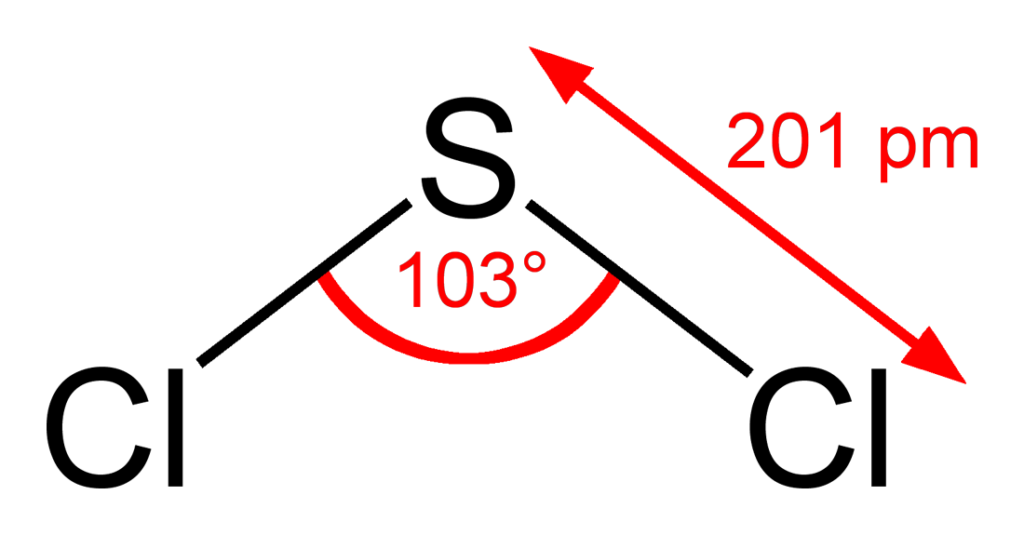
Preparation of Sulfur Dichloride

Sulfur Dichloride: Fifty grams of sulfur monochloride are placed in the distilling flask. About 0.1 g of iron powder is added to the liquid as a halogen carrier and dry chlorine is led in steadily (3-4 bubbles/sec) for one-half hour. The dark liquid that is produced is allowed to stand one hour and then one millilitre of phosphorus trichloride is added.
The product is fractionated through a small column packed with glass and the portion that boils at 55-62°C is collected. This fraction is redistilled as before from a few drops of phosphorus trichloride and the pure sulfur dichloride is collected at 59-61°C. Yield about 55 g.
The deep-red material is stable for several days at room temperature in the presence of a trace of phosphorus trichloride; it then slowly decomposes into chlorine and sulfur monochloride and may be re-purified by distillation as described.
With water, the dichloride forms sulfuric acid, sulfur, and a mixture of thionic acids, H2SxOy. Sulfur dichloride soluble in hexane, carbon tetrachloride, carbon disulfide, and ethylene dichloride.