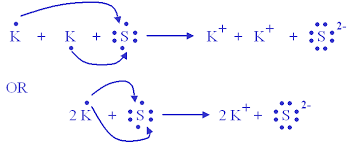
Synthesis of Potassium Sulfide
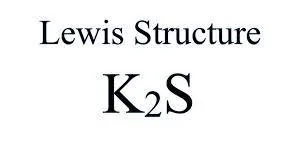
Preparation of potassium sulfide
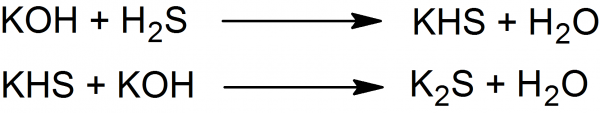
By treating potassium hydroxide with an excess of hydrogen sulfide potassium hydrosulfide (KHS) forms, which with further treatment with the same quantity of potassium hydroxide yields potassium sulfide:
Hydrogen sulfide is passed through 3 parts of concentrated potassium hydroxide solution until no further absorption takes place. Then 2 parts of concentrated potassium hydroxide solution is added in order to neutralize potassium hydrosulfide.
The rule, however, is to add only two-thirds of the quantity of solution of potassium hydroxide, as it is better the preparation should contain a little potassium hydrosulfide than that free potassium hydroxide should be present. PS should be well protected from air and moisture.
Laboratory manual of inorganic preparations, by H. T. Vulte, 43, 1895