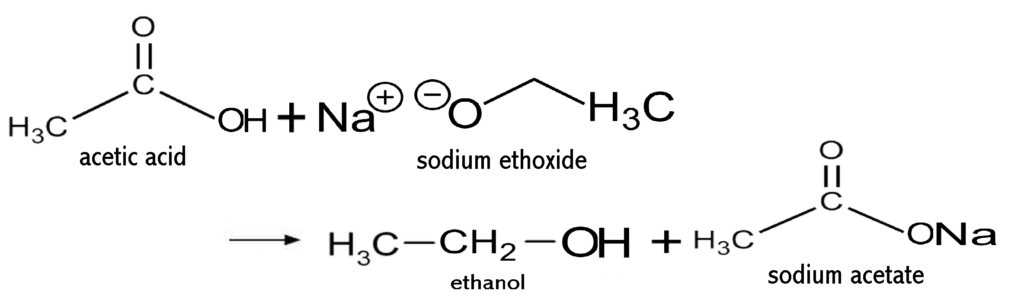
Synthesis of Sodium Ethoxide
A solution of sodium ethoxide was prepared by adding freshly cut sodium (2.72 g, 118 mmoles) to absolute ethanol (350 ml). Guanidine hydrochloride (11.3 g, 118 mmoles) was added and the mixture was stirred and heated to reflux under a nitrogen atmosphere. A solution of ethyl 7-bromo-3,4-dihydro-2-hydroxy-1-naphthoate (11.72 g, 39.4 mmoles) in absolute ethanol (75 ml) was added dropwise over 2.5 hours.
Refluxing was continued for an additional 21.5 hours, after which time the mixture was cooled to room temperature and filtered. Ethanol was evaporated under reduced pressure to give a yellow-colored foam which was dissolved in 0.1N NaOH (100 ml). The basic solution was extracted with ether and neutralized with acetic acid: water (1:9) to cause precipitation of the product.
The precipitate was collected, washed with water and ether, and dried to give 3-amino-9-bromo-5,6-dihydrobenzo[f]quinazolin-1(2H)-one as an off-white powdery solid. (6.45 g, 53%) 1H NMR (DMSO-d6 , 80 MHz) δ: 2.45-2.90(m, 4H, CH2CH2); 6.80(br s, 2H, NH2); 7.03-7.29(m, 2H, ArH); 8.60-8.75(m, 1H, Ar); 11.0(br s, 1H, NH). Anal. Calculated for C12H10BrN3O.H2O C, 46.47; H, 3.90; Br, 25.76; N, 13.55. Found: C, 46.58; H, 3.85; N, 13.60; Br, 25.87.
Literature source US05166174
Similar syntheses