Preparation of Phosphorus Pentachloride
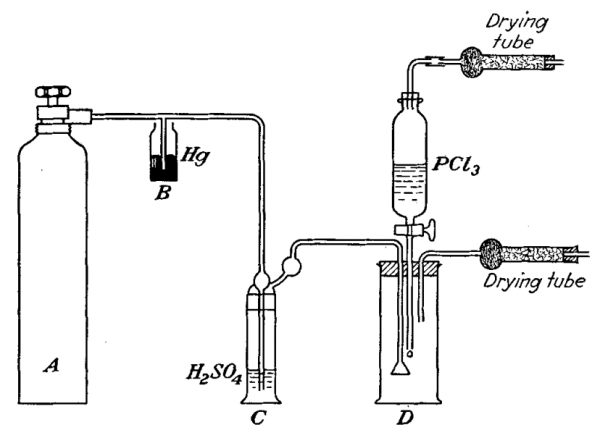
The direct reaction of phosphorus trichloride and chlorine under controlled conditions provides a simple method for preparing a high grade and yield of phosphorus pentachloride. A glass apparatus for the synthesis of Phosphorus Pentachloride is shown below.
A is a tank of chlorine, however a chlorine prepared by reaction of potassium dichromate and hydrochloric acid also could be used. B, a safety bottle; C, a bubbling bottle to observe rate of flow of chlorine gas, bottle C also serves for drying Cl2; and D, a 2 lite reaction flask – neckless jar fitted with a three-hole stopper. Through one hole is placed glass tube for introduction of chlorine, a dropping funnel is passed through the second hole.
The content of dropping funnel (phosphorus trichloride) is protected from air moisture with the drying tube. The third hole contains an outlet tube for excess chlorine. It is also protected from air moisture with the drying tube. 50 g of phosphorus trichloride are placed the dropping funnel and steady stream of chlorine is passed through the apparatus until the reaction vessel is completely filled with chlorine.
Phosphorus trichloride is added to the reaction vessel at a rate of about one drop every second. If the rate of addition of phosphorus trichloride is to slow phosphorus pentachloride will form in the dropping tube and can block it. When the phosphorus trichloride has been added, a stream of chlorine is continued for additional 5 minutes.
Then the reaction mixture is set to stand for 30 min in order to chlorinate the last traces of phosphorus trichloride. The reaction product – PP is scrape out into a dry glass-stoppered bottle. The yield of phosphorus pentachloride is 85%. Phosphorus pentachloride melts at 148° C and sublimes at 160°C. Phosphorus pentachloride is soluble in carbon disulfide and benzoyl chloride.
Inorganic Syntheses, R. N. Maxson, Volume I, 99-100, 1939

